Properties of Water
So, you're thinking of making a planet with significantly different quantities of water than Earth. Maybe there's less of it, or none at all. Maybe you want to spice things up with a different liquid medium. If you're planning to play around with water, it's necessary to understand why water is important to the life we currently know.
Water is a cornerstone of life on Earth. You may have heard that your body is made up of anywhere between 50% and 70% water. This amount can flucuate over time, but in a healthy human the amount remains above 50%. Water is also a major component in the bodies of animals, plants, and fungi. Cells themselves are filled with cytosol, a water-based solution that surrounds organelles and hosts many metabolic reactions. Access to water is considered necessary for all forms of life on Earth, and it is used in almost every biological process.
Water is also considered key to the origin of life on Earth. The earliest organisms not only used water to function, but lived in aquatic environments.
What makes water unique?
To answer this, we'll need to do a little chemistry. Atoms are the building blocks of everything in our universe, and life is no exception. Unless your alien planet exists in another universe with different laws of physics, your organisms will also play by the rules of chemistry.
On a molecular level, water is the combination of two hydrogen atoms and one oxygen atoms, as expressed by its chemical formula H2O. These atoms are held together by a covalent bond, which essentially means that they group together to share their electrons. Simply put, this is because of the number of electrons in each element. Hydrogen only has one electron but prefers to have two, while oxygen has eight electrons but prefers to have ten. The oxygen shares one of its electrons with each hydrogen, while the hydrogens share their own electrons with the oxygen atom. They can't all have their preferred number of electrons at the same time, but electrons are constantly moving very quickly between the atoms, so the hydrogens can sometimes have two electrons in their orbits and the oxygen can sometimes have ten electrons in its orbit. Think of it as a chemical compromise.
It looks like this:
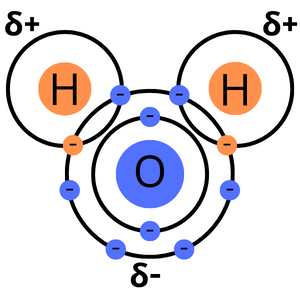
You may notice those funky little symbols (they're Greek lowercase deltas δ) with plus and minus signs. Those tell us the charge that each individual atom has in the water molecule. Oxygen attracts those extra electrons a little better than hydrogen does, which means that the oxygen "side" of the molecule has a negative charge, while the hydrogen "sides" have a positive charge. This is key to water's unique property: hydrogen bonding.
Hydrogen bonding occurs when two water molecules stick together. You're probably aware that positive and negative charges are attracted to one another. In this case, a hydrogen atom from one water molecule sticks onto the oxygen atom of another molecule. Given enough of these molecules, hydrogen bonds become extremely frequent. These bonds give water the cohesion that lets them form droplets or produce surface tension. However, these bonds are also extremely weak, which is what allows water molecules to "break" tension and slide past one another, making them an easy liquid to manipulate and move through.
Water can also use its charges to attract other charged molecules, which causes many substances to dissolve easily in water. This property makes water a universal solvent. This is important, as it allows water to move other molecules with it. This is how water moves vital minerals and nutrients through the bodies of organisms. Without water, it would be much harder, perhaps impossible, for these substances to move throughout the body. Its dissolving powers also enable water to carry other chemicals throughout the environment, seeping into the ground or racing along ocean currents.
Does my planet need water for life to exist?
It's hard to say. No life on Earth has been observed to be capable of existence without ever using water in some way. We haven't discovered any substances that dissolve molecules as effectively as water does.
However, there is some speculation on this topic. Some scientists have proposed that life could exist in the liquid methane (CH4) and ethane (C2H6) of the Saturn moon Titan. Their respiration would utilize an entirely different set of molecules than life on Earth. Like water, both methane and ethane are colorless in liquid form, so these alternatives would not affect the potential sight of organisms inhabiting them. It is uncertain if these organisms could exist without a substance more similar to water or a radically different method of transporting chemicals through their bodies, since methane and ethane are not similar to water as solvents.
Another top candidate is liquid ammonia (NH3). Liquid ammonia has many similar chemical properties to water, including the ability to dissolve many organic molecules. A potential difficulty with ammonia is that its boiling point is -28F (-33C), so this substance would require life to develop on either a very cold planet, or on a planet with an extremely strong atmospheric pressure to raise the boiling point to a more moderate temperature. Liquid ammonia is clear, but dissolving certain substances can cause it to take on a blue or yellow hue.
Other options have been explored to varying degrees. Other contenders include hydrogen fluoride (HF), hydrogen cyanide (HCN), and hydrogen sulfide (H2S). Each presents its own challenges, but may be best suited to planets with circumstances extremely different from Earth such as (depending on the specific substance) extreme cold, little to no seasonal difference or day-night temperature change, high or low atmosphere density, and high concentrations of cosmically rare substances.
What if my planet has water, but there's more or less water than on Earth?
If you are creating water-based lifeforms on a planet with more than trace amounts of liquid water, then water scarcity or abundance is unlikely to radically alter your creatures on an atomic level in the same way that having no access to water would. A future article will explore the macro-scale effects of having a particularly wet or dry planet.